Two-dimensional Porphyrin-Fullerene Networks
The unique electrochemical and photophysical properties
of porphyrin
and [60]fullerene
(C60) compounds make them promising candidates for the
construction of two- and three-dimensional organic-based materials.
Metallo-porphyrins and their derivatives have been shown to be exceedingly
useful building blocks for the construction of 3D supramolecular functional
networks due to their excellent thermal and chemical stability and synthetic
versatility. Materials built from porhyrins could be interesting for
various potential applications such as catalysts, molecular sieves,
or chemical sensors.
|
|
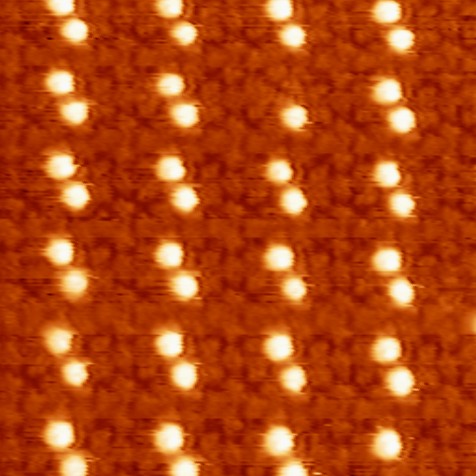
Figure 1. STM image of porphyrin–C60 assembly
(scan range: 30x30 nm2, It=16 pA, Vbias=2.67 V); the blue circle indicates
a C60 vacancy. |
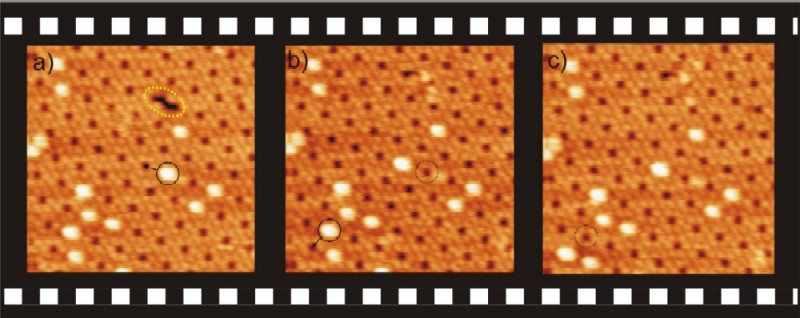 |
Figure 2. a),c),d) Three sequential STM images
(scan range: 30 × 30 nm2, Vbias = 2.9 V, It = 12 pA, T = 298 K;
the time difference between each image is 62 s). b) Proposed molecular
model of a C60 molecule hosted inside a supramolecular porphyrin-based
pore. Driven by thermal fluctuations, single C60 ad-molecules (solid
circle) are able to displace to neighboring pores as time proceeds.
At the same time, bright–dim fluctuations (colored arrows) of
single porphyrin molecules propagate through the porous network indicating
conformational motion of the 3,5-di(tert-butyl)phenyl moieties. Self-repairing
of a defect in the porous structure (dashed ellipse in image a) is also
observed. |
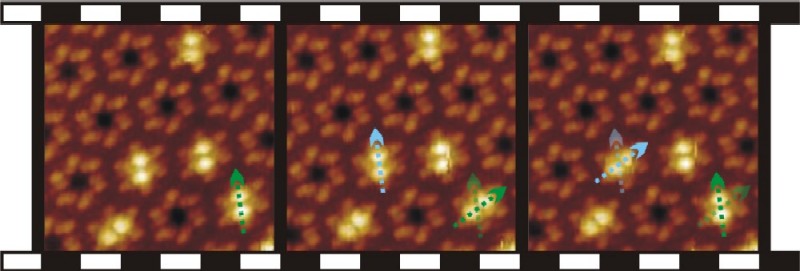 |
Figure 3: A chiral nanoporous porphyrin
network as host for second layer porphyrin guests. The guests can be
found in three distinguishable positions (only two shown). At temperatures
above 110 K they switch randomly between these positions. At lower temperatures,
the switching can be induced with an STM tip. As the guests do not hop
between pores, this is an example of intraporous hopping. |