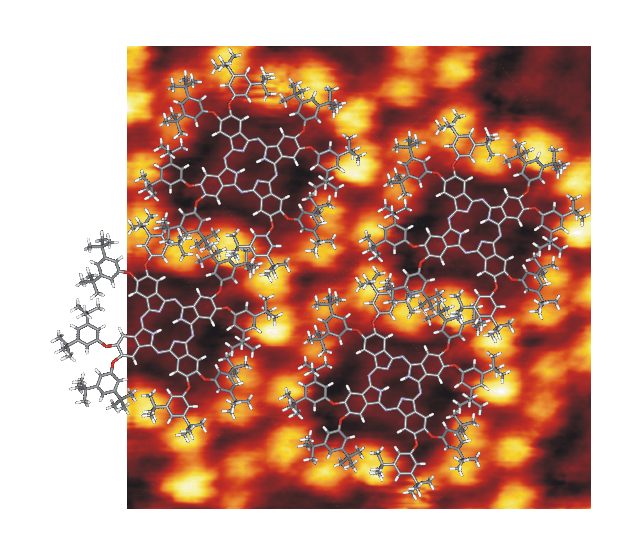
Ordered patterns of a phthalocyanine derivative
on metal surfaces
Phthalocyanines
have been the subject of intense studies, due to their wide range of
optical, structural and electronic properties arising from their large
pi-conjugated system and their assembling into co facially-stacked arrays.
Furthermore, at the peripheral binding sites a variety of magnetic,
electronic, redox and photo-active components could be attached.
We study a phthalocyanine derivative which contains eight ditertbutylphenyl
substituents. Thin films of the derivative are prepared in a UHV setup
on metal crystals by evaporation. The investigations are carried out
with a homebuilt STM at room temperature.
The investigated derivative is considered as a precursor for the examination
of other functionalized phthalocyanine derivatives. Particularly, the
tetrathiafulvalene-annulated phthalocyanine is an interesting candidate,
because of its redox properties. [1]
[1] C. Loosli, C. Jia, M. Haas, M. Dias, E. Levillain, S.-X. Liu, A.
Hauser, S. Decurtins,
J. Org. Chem., 2005, 70(13), 4988-4992
|